What Is Glaze?
After you shape and bisque fire a clay piece, you usually coat it in glaze before firing again. A glaze is a glass formula tuned to melt and fuse onto clay at certain temperatures. It seals the ware, boosts durability, and adds color or texture. Without glaze, many pieces would leak, stain, or wear quickly.
People have made unglazed ceramics for more than ten thousand years, but glazing arrived later. Early potters found that wood or plant ash, soda, lead, and other alkaline minerals would melt in the kiln and seal a pot. Civilizations in the Middle East, China, and Egypt refined these discoveries as kilns improved.

From left to right: Ash glazed Storage Jar, China, 206 B.C.E.–220 C.E. Lead glazed Miniature Ewer, Iran, 11th–12th century High-fire Celadon Ewer, China, 11th–12th century
In ancient China and elsewhere, alkaline/ash glazes arose from the fluxing oxides naturally found in wood ash, which helped silica melt into a stable glass coating at high temperatures. Lead glazes, meanwhile, were once very common in low-fire traditions but are no longer advised for functional ware due to toxicity. By refining fluxes and strengthening the clay body itself, potters in China eventually perfected high-fire stoneware and porcelain glazes, which greatly influenced later ceramic traditions worldwide.
Since the late 19th century, ceramicists have used chemistry to explain and design glazes: measurable oxide formulas plus intentional firing schedules. Blending craft and science lets us tune color, texture, gloss, and glaze fit with far more control.
The Composition of a Glaze
Before firing, glaze ingredients are a dull, powdery layer on the clay body. In the kiln they melt and fuse into a thin, glassy coating.
Most glazes boil down to three oxide roles: glass former, flux, and stabilizer. Silica (SiO₂) is the common glass former but needs very high heat on its own. Fluxes (sodium, potassium, calcium, boron oxides) lower that melting point. Stabilizers (often alumina from clay materials like kaolin) thicken the melt so it doesn’t run off and stays durable once cooled.
Although these three groups (glass former, flux, and stabilizer) are the heart of every glaze, many other materials also come into play. Colorants such as iron, cobalt, and copper oxides shift the glaze’s hue or create special visual effects, while opacifiers like tin oxide and zircon introduce opacity. In modern pottery, glazes can be designed in a seemingly infinite variety of ways, sometimes with as few as two ingredients or as many as a dozen or more.
To learn more about these oxide groups and how they function in glazes, see Major Oxides in Glazes.

The combination of just two ingredients, Chinese Glaze Stone and Glaze Ash, provides all the necessary components for a stable and durable glaze. Glaze Stone provides the glass former, stabilizer, and some alkaline flux, while the glaze ash provides flux in the form of CaO and minor amounts of MgO. Glaze Link
Colorant example: A line blend adding the colorant Cobalt Oxide in 0.1% increments to a glaze.
Eutectics: How Oxides Interact
Eutectics let mixes of oxides melt at lower temperatures than any single oxide alone. The melt isn’t linear: adding more flux like whiting (CaO) doesn’t just keep lowering the melting point. Instead, certain ratios give a dramatic drop—the eutectic point. Hitting that “sweet spot” lets you formulate efficiently without piling on flux.
Phase Diagrams map how oxide combinations melt across temperatures and show the eutectic point. You’ll see two-oxide (binary) or three-oxide (ternary) diagrams (like SiO₂-Al₂O₃-CaO). They look technical but function like road maps to stable, predictable glazes with less trial and error.
Eutectics are part of everyday life. For example, when you sprinkle salt on icy sidewalks, the salt lowers the freezing point of water, causing the ice to melt. This is similar to how eutectics work in glazes.
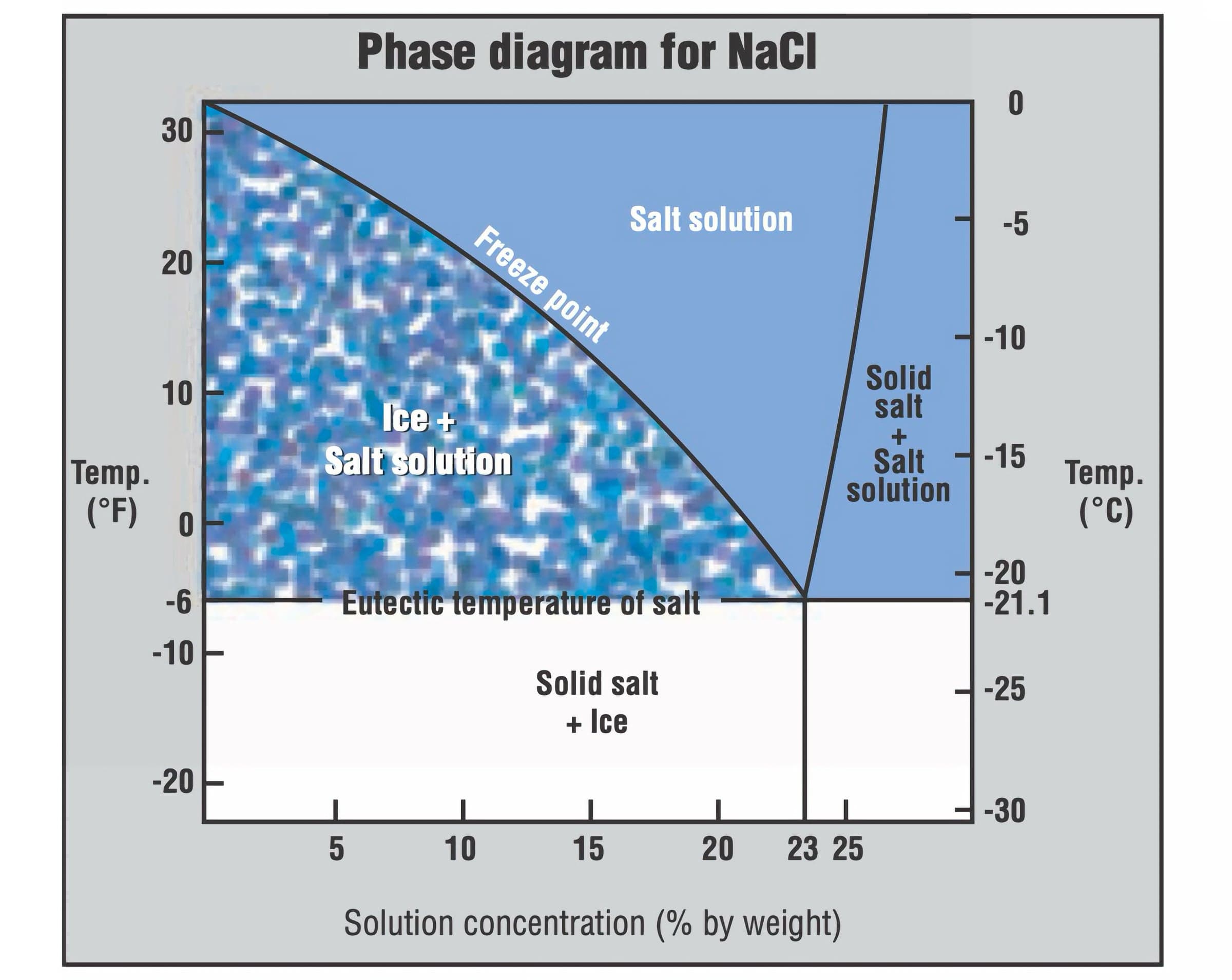
This phase diagram illustrates the impact of NaCl concentration and temperature on the phase of an aqueous NaCl solution. Salting Roads “Salting Roads” by DR Kimbrough, American Chemical Society
Melting & Fusing
When fluxes interact with silica and alumina at high temperatures, the mixture becomes a molten glass that flows and can combine at the surface of the clay. The proportions of silica, alumina, and flux determine how easily the glaze melts, how fluid it becomes in its molten state, and how durable or glossy it remains after cooling. Some fluxes, such as sodium and potassium, encourage higher thermal expansion in the finished glaze, while materials like dolomite (which supplies magnesium) can produce a lower-expansion, potentially more matte surface through crystalline growth.

Reflected light photomicrograph of a cross-section of a sherd showing the layers of ceramic body, slip, and glaze. The Cyprus Institute
There are several firing stages where the glaze undergoes key transformations. At lower temperatures, moisture and other organics burn off, making the clay and glaze safe from steam explosions or smoke. As the kiln continues to heat, the glaze particles begin to sinter (meaning they partially melt and fuse together) until they become fully molten at peak temperature. At this stage, the glaze actively interacts with the clay surface, often forming an interfacial layer that locks the glaze onto the body. Cooling then solidifies the molten glass. If under-fired, the glaze won’t fully melt and may remain rough or matte in unintended ways. Conversely, over-firing can lead to defects like blistering or pinholing, and if the clay body itself is overheated, it can deform or even begin to fuse.
Bisque and Glaze Application
Many ceramicists prefer bisque firing first, which turns the clay into a porous yet firm body that readily absorbs water from the glaze slurry. This absorption helps the powdery glaze ingredients adhere evenly. In contrast, a technique called raw glazing or “once-firing” applies glaze to unfired clay. Once-firing can save time and energy but demands precise control of moisture levels to prevent the glaze or the body from failing during the single firing.
Glaze Firing Stages
- Burnoff (Low Temp): Residual moisture and organics combust.
- Sintering & Melting: The glaze particles start to fuse. By peak temperature, the glaze is fully molten and can interact with the clay surface.
- Bonding: A thin interface layer forms between clay and glaze, improving adhesion.
- Cooling & Solidification: As the kiln cools, the molten layer solidifies into glass.
Each of these phases influences the final outcome, and minute adjustments in time, temperature, and atmosphere can change a glaze’s color, texture, or durability dramatically.
Learn more about Bisque & Glaze Firing
Cone Firing Temperatures
Ceramicists measure heatwork using pyrometric cones, which melt and bend once a specific balance of temperature and time is achieved. Ceramic wares fall broadly into three popular firing ranges:
- Low-Fire (Earthenware): ~Cone 06–02 (~1800–2050 °F / 980–1120 °C). Glazes here often rely on high flux content to melt at lower temps.
- Mid-Fire (Stoneware): ~Cone 5–6 (~2160–2230 °F / 1180–1220 °C). Popular for functional ware; offers broad color possibilities.
- High-Fire (Stoneware & Porcelain): ~Cone 8–11 (~2300–2380 °F / 1260–1300 °C). Produces very durable, sometimes more subtle, glazes that can vary dramatically in reduction firings.
It is essential to match the glaze’s firing range to the clay body’s maturation temperature. If the body is designed to mature at cone 6–8, for instance, it usually pairs well with a cone 6 glaze recipe. Mismatch here can lead to problems like bloating, incomplete vitrification, crazing, or shivering.
Learn more about Temperature and Heatwork
Firing Atmosphere & Cooling

Examples of different firing atmospheres: From left to right: Salt-glazed Jug, 17th century, German , Reduction-fired Funerary jar, China, 12th–13th century , Ash-glaze Tea Caddy, Japan, 17th century , Raku Tea Bowl, Japan, ca. 1820-1830
- Oxidation: Plenty of oxygen (typical of electric kilns). Metallic colorants stay in higher oxide states and often give bright, consistent colors.
- Reduction: Oxygen-starved (common with gas or wood kilns). Colorants like iron or copper shift chemistry, yielding effects like copper red or celadon and often changing melt fluidity or crystallization.
- Neutral: Atmosphere that’s neither clearly oxidizing nor reducing.
- Salt & Soda: Vapor glazing from salt or soda ash introduced into the kiln, creating rivulets and orange-peel textures.
- Wood: Fuel introduces ash that lands on ware and melts into the surface.
- Raku: Removal from the hot kiln and rapid cooling for dramatic effects.
- Luster: Metallic/iridescent overglaze fired at low temperature (silver, copper, bismuth, etc.).
Kiln Cooling
Even a single glaze can appear very different in oxidation vs. reduction, or under varying cooling schedules. Once the maximum temperature is reached, how quickly the kiln cools can dramatically affect the final surface.
- Fast Cooling: Tends to “freeze” the molten glaze quickly, preserving a bright, glassy surface.
- Slow Cooling (or Soaks): Allows crystals to form and grow, which can convert a once-glossy glaze into a satin or matte finish. Some glazes specifically require slow cooling or an extended soak to develop surface crystals or special color effects.

Example of cooling’s effect on a glaze: Left: Teadust glaze with slow cooling. After reaching cone 10, immediately close up kiln entirely. Right: Teadust glaze in fast cool to 1000°C. After reaching 1000°C, completely close up kiln.
Learn more about Cooling: Natural vs. Programmed
Glaze Characteristics
Glazes can be categorized in a variety of ways, but two major factors are transparency and surface finish.
Transparency
Glazes range from fully transparent (like a clear window) to heavily opaque (e.g., white tin glaze). Opacity can come from additives (tin oxide, zircon) or from crystalline inclusions that scatter light.

Line blend adding Zircopax in percentages from 0-10% to a clear glaze to adjust transparency.
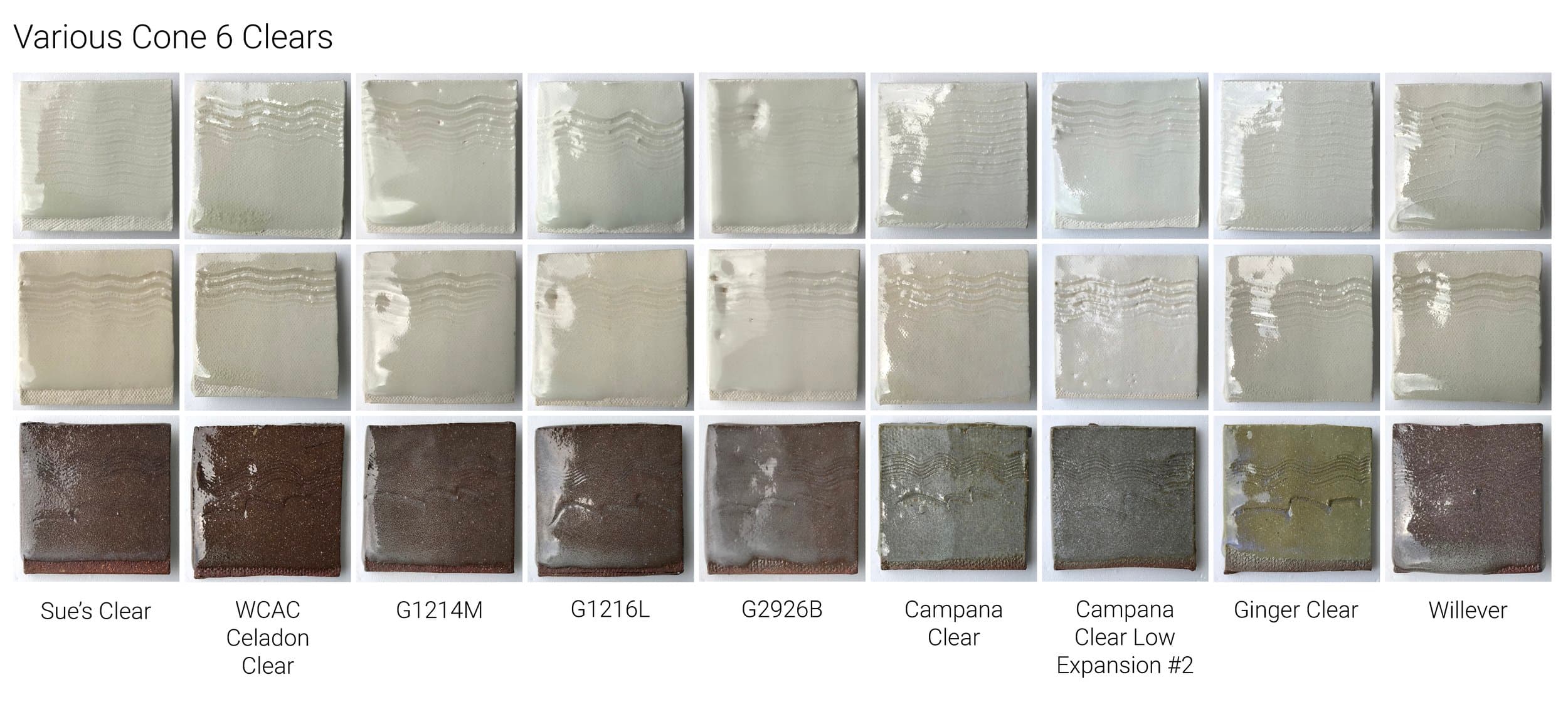
Examples of cone 6 clear glazes showing a range of transparencies.
Surface
Glazes also differ in how much they reflect light.
- Glossy: Highly reflective, smooth. Often easy to clean.
- Semi-matte or Satin: A subdued sheen partway between gloss and matte.
- Matte: Dull surface, sometimes slightly textured at the microscopic level due to devitrification or crystal growth. Always verify matte glazes for food-safety if they contain unusual ingredients.

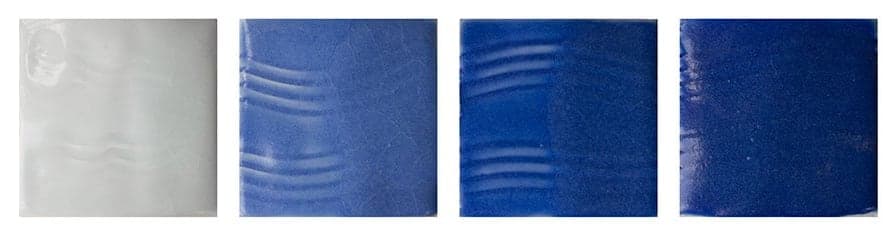
Examples showing the effect of varying Silica:Alumina ratio on glaze surface.
Glaze Fit and Thermal Expansion
When the piece cools, both glaze and clay body cool and shrink. If their rates of thermal expansion and contraction are mismatched, the glaze may craze (forming a network of fine cracks) if it shrinks too much relative to the body, or it may shiver and flake off if it doesn’t shrink enough. This relationship is sometimes measured using a calculated thermal expansion (CTE). Having a glaze and clay body in harmony (often called “good glaze fit”) is essential for producing sturdy, long-lasting pottery without defects like crazing or shivering.
You can read more about these issues and their potential solutions in Common Glaze Defects
References
- Ceramic Glaze (Wikipedia)
- Ceramic Glaze (Digitalfire)
- Dave Finkelnburg, “How Glazes Melt” (NCECA 2012)
- “UMF Phase Diagrams: Guidemaps for Ceramic Glaze Development” by Howard Sawhill
- “Phase and Eutectics” by Linda Bloomfield
Books you can borrow online:
- The ceramic spectrum by Robin Hopper
- Chinese glazes : their origins, chemistry and re-creation by Nigel Wood
- The craft and art of clay by Susan Peterson
- Clay and glazes for the potter by Daniel Rhodes
- Cooper’s book of glaze recipes by Emmanuel Cooper
- Glazes for the potter by Emmanuel Cooper
- The potter’s book of glaze recipes by Emmanuel Cooper
- Electric kiln ceramics by Richard Zakin
- Glazes cone 6 : 1240⁰c/2264⁰f by Michael Bailey
- A handbook of pottery glazes by David Green
- Salt-Glaze Ceramics by Rosemary Cochrane
- Mastering raku by Steven Branfman
- Celadon Blues by Robert Tichane
- Chinese stoneware glazes by Joseph Grebanier
- Dry Glazes by Jeremy Jernegan
- Cone 5-6 glazes : materials and recipes
- The ceramic glaze handbook : materials, techniques, formulas by Mark Burleson